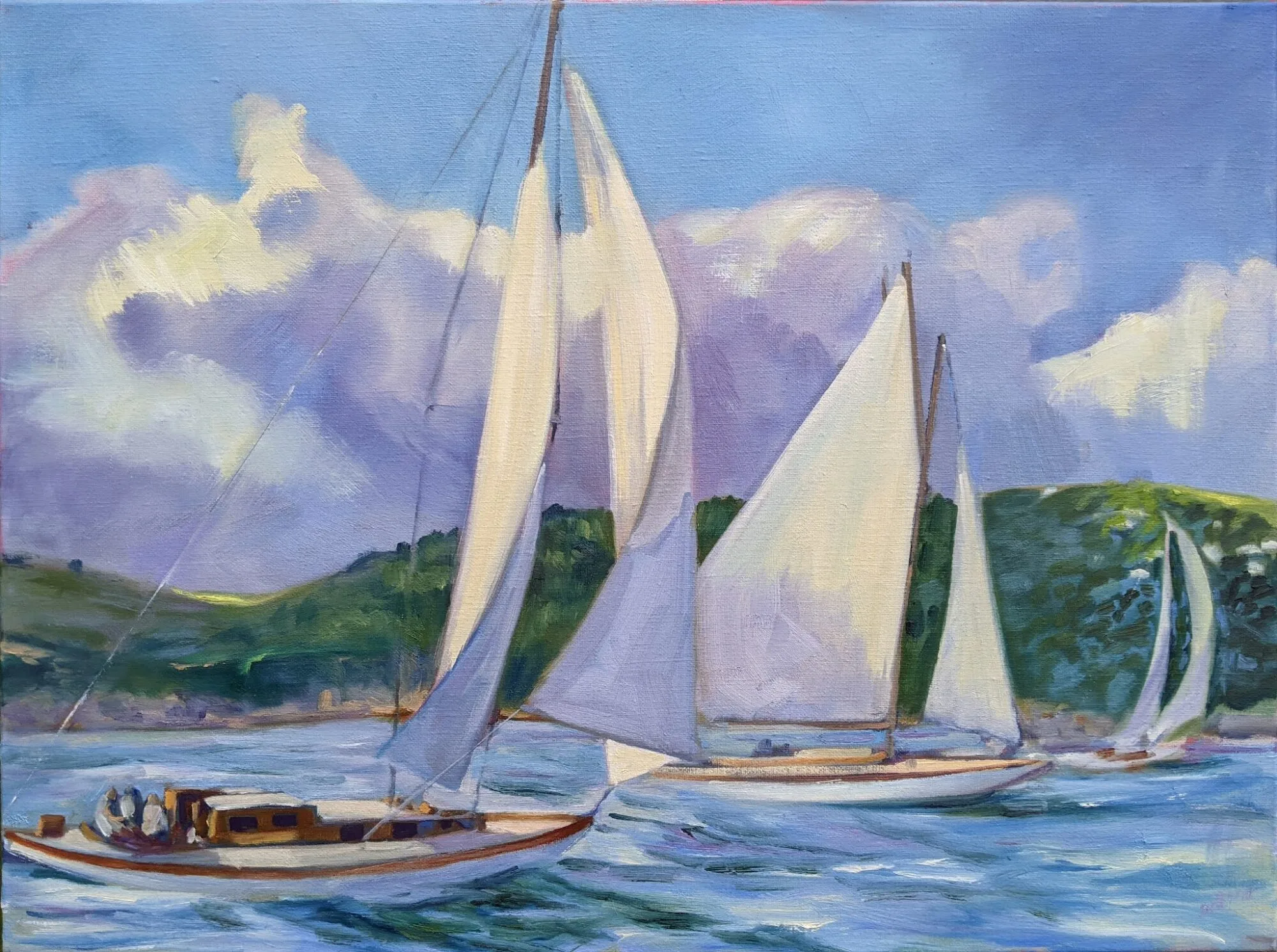Yes, the ‘fat over lean’ rule still pertains in oil painting.
 |
| Estuary Light, by Gwen Sylvester. |
Gwen Sylvester’s Estuary Light, above, won this year’s Juror’s Choice Award at Wet Paint on the ‘Weskeag. It is a perfect application of acrylic paint: luminous, fluid, but not washed out into faux watercolor. The nominal subject—egrets—are just suggested in the midfield.
Kay Sullivan and Gwen and I sat together to watch the auction. We are the last three years’ winners of the Juror’s Choice Award. The art world is one of the last strongholds of gender bias, intentional or not. To have three women winners in a row is an anomaly, and speaks well of this event.
A student at my workshop last week asked me whether the ‘fat over lean’ rule still applies now that we’re using odorless mineral spirits (OMS) instead of turpentine. It’s a great, complicated question. Turpentine is pine-tree essential oil. OMS are petroleum distillates. But before you rush back to using turpentine because it sounds “natural,” it is linked to a host of respiratory and other illnesses.
 |
| A lobster pound at Tenant’s Harbor, was my entry into Wet Paint on the ‘Weskeag. |
I saw conservator Lauren Lewis at the auction. She told me the answer to Maureen’s question was still yes. That was all we had time for, so the rest of this explanation comes from the internet.
Solvents like OMS or turpentine dry through simple evaporation. The binder oils in paints are more complex; they cure by something called crosslink polymerization.
A polymer is a long chain formed by many little molecules that stick together. A crosslinked polymer network is a mesh of these chains that are woven together. Just as with fabric, woven threads are stronger than individual fibers. Crosslinked polymers can be very strong.
These crosslinked polymers also resist solvents better than simple chains. To dissolve a polymer, each of the long molecules must be surrounded by the solvent and dispersed. As the polymer gets larger and larger, it becomes more difficult to dissolve the polymer molecules.
Sometimes there are enough links to connect all of the polymer molecules into a single mesh. When this happens, you can no longer dissolve the polymer molecules—they either float away as a single lump of paint or they don’t go at all.
| You can’t reopen half-dry oil paints like you can half-dry acrylics. That’s because of crosslinked polymer chains. |
Think about the last time you let your paint dry up on your palette. You can’t ‘open’ half-set oil paint like you can with partly-set acrylics. All the pigment is drawn into the lumps. That same tendency is what makes them so tough on the canvas, and why you want that oily layer on the surface.
Humidity often damages a paint film. This happens when water molecules surround the paint components and push them around. Crosslinks limit how much water can get into the film.
Speaking of humidity, I leave in a few minutes for Saranac Lake, NY, for the Adirondack Plein Air Festival. From there I go to Plein Air Plus in Long Beach Island, NJ. As I’m writing this, cool, damp air is rushing in my window. My house sits above Rockport harbor. The ocean is my air-conditioner. Why do I ever leave in the summer?